A changing landscape
Over recent years, the regulatory landscape for topicals has changed. Whether it’s a shift in speaking about products qualitatively, having higher expectations when matching a generic product to an originator, or using different performance parameters, Gloria Ho, Global Technical Marketing Manager at BASF, says the shift is due to a greater emphasis on safety and efficacy.
“Historically, oral-based products have undergone higher levels of scrutiny than topical-based products due to grandfathered regulations. We’re now seeing that both end users and regulatory bodies are looking for that additional level of quality and trust,” says Ho. “The field is more science-driven and is becoming a more regulated industry than it previously had been if we looked back 20 or 30 years.”
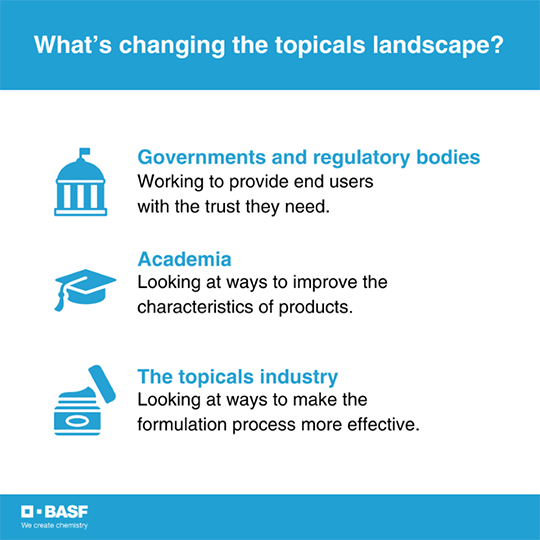
There are several factors driving this change.
First, governments and regulatory bodies are working to provide end users with the trust they need to know that finished formulations have undergone strict regulatory requirements.
Academia is another driver. “We’re seeing specialists looking at different ways they can improve the characterization of topical products,” Ho continues. “By understanding how to categorize products with certain techniques, and relating those to a product’s overall performance, you can better predict a product’s attributes such as physical stability or shelf life.”
A third driver is the industry itself. “Product formulators are looking for ways to make the development process more orderly. When standardized methods exist to characterize topical product attributes, then it becomes much simpler to employ a proven procedure to demonstrate product performance.”